Effect-based methods
The Water Framework Directive (WFD), which came into force in 2000, aims to ensure that all European water bodies are in at least good ecological and chemical status by 2027. Less than 10 % of Germany's surface waters currently meet this target.
It is relatively difficult to estimate the significance of pollutants in missing this target. Currently, a selection of priority substances and river-specific pollutants are measured across the board. However, a major disadvantage of classic chemical analysis is that only chemical pollutants that have been specifically searched for are found. Specified substances from the analytical measurement programs could easily be replaced in the water by other chemicals with a similar hazard potential. In practice, it is hardly possible to estimate a possible contamination by measuring all potential pollutants. In addition, most bodies of water contain a complex cocktail of chemicals whose combined effects are completely neglected in purely chemical analyses.
Effect-based methods for assessing environmental pollution offer a solution. Here, entire substance groups with similar mechanisms of action are tested and interactions and mixing effects are taken into account. Conspicuous substance groups can then be investigated more precisely and cost-effectively using chemical analyses. Furthermore, effect-based methods can contribute to a better understanding of the relationships between the chemical and ecological status of a body of water.
In vitro test systems
Microtox Assay
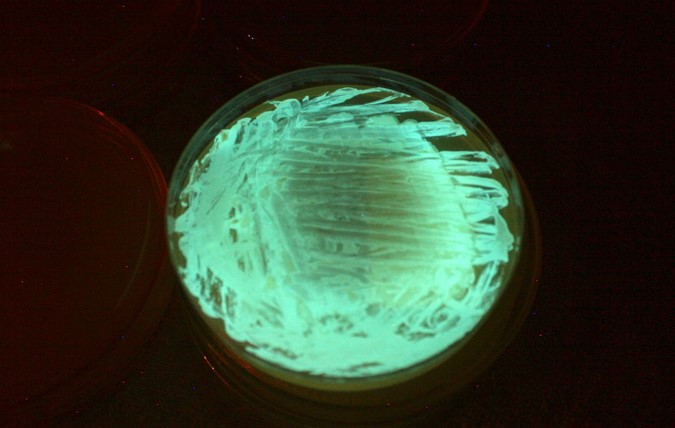
In the microtox assay, the basic toxicity of an environmental sample is tested. The bacterium used here, Aliivibrio fischeri, has natural bioluminescent properties. It is found in low concentrations in all oceans and often lives in symbiosis with other marine organisms. The bioluminescence is based on the reaction of the oxidation of luciferin to oxyluciferin by the enzyme luciferase. This reaction and thus the intensity of the luminescence can be influenced, for example, by disturbance of growth or metabolism by toxic substances. To evaluate an environmental sample, the luminescence inhibition of the bacterium is measured as a non-specific endpoint. This method is based on a standardized procedure (EN ISO 11348) and was adapted for use on microtitration plates in accordance with Escher et al (2008).
Ames fluctuation test
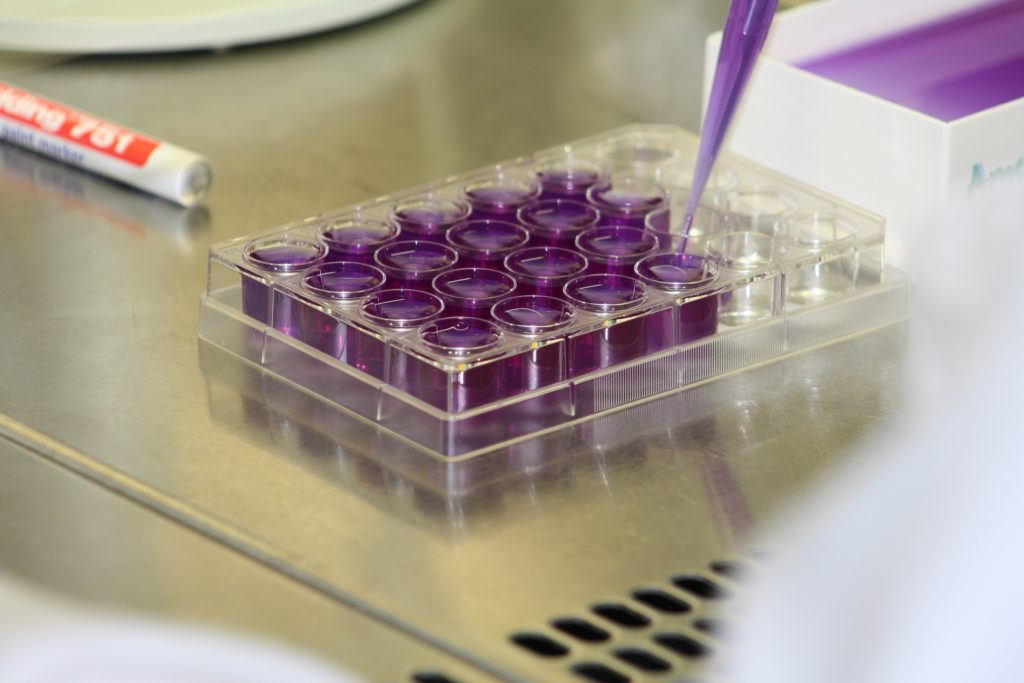
This assay can be used to detect mutagenic effects in environmental samples. Genetically modified strains of Salmonella typhimurium are used. Due to a mutation in the histidinoperone, these strains are unable to produce the amino acid histidine. In the presence of mutagenic substances in the tested environmental sample, the probability of a reverse mutation increases. The number of back-mutated replicates in a sample can be detected using a color reaction. The test procedure is based on Guideline ISO 11350, but has been modified for use in microtitration plates.
Yeast reporter gene assay
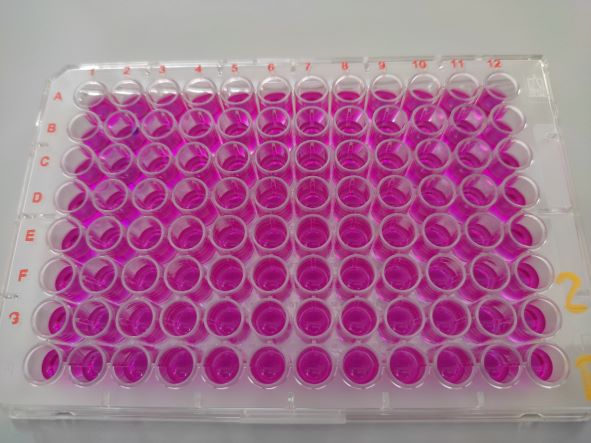
The yeast reporter gene assay is used to detect endocrine and dioxin-like substances. Depending on the endpoint sought, genetically modified strains of Saccharomyces cerevisiae baker's yeast are used in which human hormone receptors have been incorporated. In the presence of oestrogenic, androgenic and dioxin-like substances in the sample, the corresponding agonist binds to the receptor, which can be detected by fluorescence measurement. When testing anti-oestrogenic or anti-androgenic substances, a corresponding antagonistic reaction is measured.

AREc32 Assay
This test is used to determine substances in a sample that trigger oxidative stress or cytotoxicity in cells. Defense mechanisms in the cell are measured, which are induced at the cellular level during oxidative stress and thus trigger the transcription and expression of certain genes. In the AREc32 cell line used in this test, such a reaction can be quantitatively determined by measuring luminescence. Furthermore, a possible cytotoxicity of substances can be determined by checking the metabolic performance of the sites after addition of the sample
In vivo test systems
Potamopyrgus antipodarum
Due to its sensitivity to reprotoxic substances, including endocrine disruptors (EDC), P. antipodarum is suitable as an indicator for anthropogenic trace substances from wastewater from sewage treatment plants. For the investigations, snails with a shell length between 3.5 and 4.5 mm were used both as part of active monitoring in the field and in parallel exposed to a combined sediment-water system of the sampling sites in laboratory experiments.


Gammarus fossarum
Various studies have shown that the freshwater amphipod G. fossarum is sensitive to toxic and endocrine disrupting substances. In addition, they play a central role in the food web as decomposing invertebrates (shredders) and as a food source for other aquatic organisms. To ensure that only animals capable of reproduction were tested, organisms with a size of at least 6 mm were used. Here too, both active monitoring in the field and laboratory tests with native sediment and water samples were carried out.
Fish egg test with Danio rerio
The fish embryo toxicity test, or fish egg test for short, is used to detect embryotoxic and teratogenic effects on the zebrafish(Danio rerio). Teratogenic effects are defined as the impairment of embryonic development due to exposure to chemicals. The use of this organism for bioassays has numerous advantages such as easy cultivation, high reproduction rates and a transparent chorion. This allows all important developmental stages of the organism and variations within these stages to be observed. When testing water/wastewater samples, the test design and procedure is based on DIN EN ISO 15088.


Macrophytes
In addition to producing biomass, plant organisms fulfill a wide variety of functions in the watercourse ecosystem (e.g. food, habitat, absorption of nutrients and pollutants as natural "filters"). They were therefore included in this project as an additional "biomonitoring group". The macrophytes Lemna gibba and Myriophyllum spicatum are used here.

Contact us
DECIDE
Dr. Matthias Oetken
Johann Wolfgang Goethe University Frankfurt
Phone: +49 (0) 69 798 42148
CONTACT
EffectMon
PD Dr. Andrea Sundermann
Senckenberg Society for Nature Research
Phone: +49 (0) 6051 61954 3124
